A2369C Current Good Manufacturing and Laboratory Practices Assignment: Pharmaceutical Manufacturing, GLP Practices, and Ethical Issues in Drug Development
Part-Time Diploma in Applied Science (Pharmaceutical Sciences)
Graded Assignment
There are THREE (3) COMPULSORY questions for this graded assignment. You must adhere to the word limits indicated for the question as required. Words more than the stipulated word limit will not be taken into consideration even if the correct answer is contained therein.
Question 1 (Total: 24 marks)
Republic Pharma company is commissioning a new manufacturing line that is based on the picture below. As part of the project team, you are tasked to contribute to the planning and documentation strategy required.
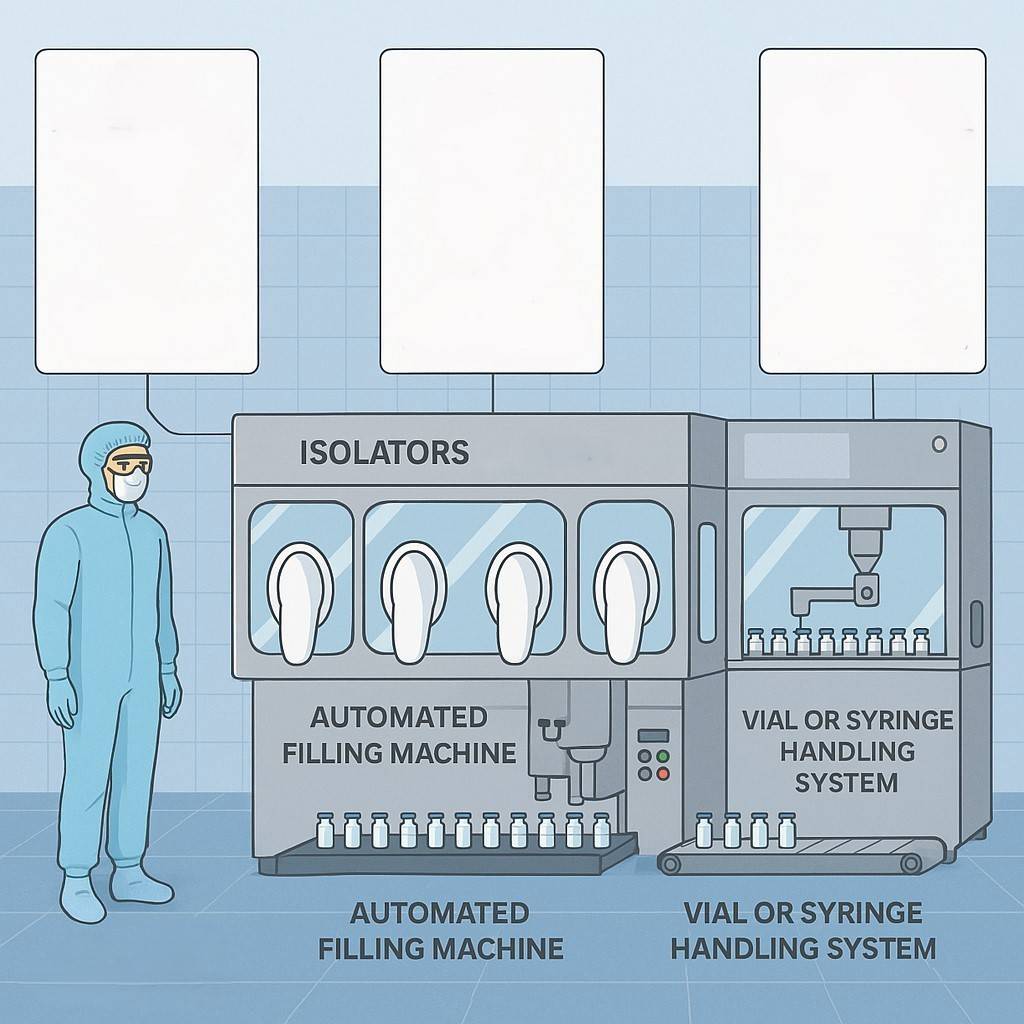
a) Identify and describe three (3) critical GMP documents needed before production can begin, explaining their purpose and key elements. (6 marks)
b) Outline the steps required to validate the production line (IQ, OQ, PQ) and describe the importance of each step. (6 marks)
c) Discuss the regulatory considerations (e.g., audits, authority approvals, registration) that must be fulfilled before commercial release. (6 marks)
d) Propose how Good Documentation Practices (GDP) will be implemented across the team to ensure traceability and compliance. (6 marks)
Question 2 (Total: 20 marks)
a) Identify and explain four (4) essential components of Good Laboratory Practice (GLP) in the context of the picture below. (8 marks)
b) Describe the role of a Quality Assurance Unit (QAU) in GLP compliance. (4 marks)
c) Create a one-page SOP outline (in bullet format) on how to maintain a laboratory notebook in line with GLP and ALCOA+ principles. Include the Title and Purpose of the SOP. (8 marks)

Question 3 (Total: 20 marks)
A research associate in Republic Pharma company published promising results about a novel drug’s efficacy.
Efficacy Data for Novel Drug “NeuroVex” Study Design:
- Randomized, double-blind, placebo-controlled trial
- Sample size: 120 patients (60 drug, 60 placebo)
- Indication: Treatment of early-stage neurodegenerative disorder
- Duration: 12 weeks
Primary Endpoint: Improvement in cognitive function score (CF Score)
Internal results recorded on laboratory notebook:
| Group | Baseline CF Score | Week 12 CF Score | Mean Change |
| NeuroVex | 45.2 | 55.0 | +9.8 |
| Placebo | 44.9 | 48.1 | +3.2 |
Results shown on published scientific article:
| Group | Baseline CF Score | Week 12 CF Score | Mean Change |
| NeuroVex | 45.2 | 62.8 | +17.6 |
| Placebo | 44.9 | 48.1 | +3.2 |
Task:
a) Compare the published results with the internal lab data. Identify and evaluate the ethical breaches that may have occurred based on the discrepancies you observe. (6 marks)
b) Discuss the potential regulatory and legal implications for the company and the researcher if these discrepancies are verified during an inspection or audit. (6 marks)
c) Propose a three-step action plan the company should take to investigate, address the situation, and prevent recurrence of such an incident. (8 marks)
Use diagrams, tables or flowcharts where relevant to support your report. (3 marks) Cite at least three references to support your recommendations. (3 marks)
(Word limit: 2000 words)
The post A2369C Current Good Manufacturing and Laboratory Practices Assignment: Pharmaceutical Manufacturing, GLP Practices, and Ethical Issues in Drug Development appeared first on Singapore Assignment Help.